In August 2018, HIQA commenced work on a health technology assessment (HTA) of a pre-exposure prophylaxis (PrEP) programme for populations at substantial risk of sexual acquisition of HIV. The HTA was requested by the Clinical Lead in Sexual Health at the HSE, and the request was subsequently endorsed by the Department of Health. Based on the available evidence, the HTA will inform decision making by the Minister for Health and the HSE.
Search Filters
HTA of a PrEP programme

Foreword
PrEP is a form of HIV prevention whereby oral anti-retrovirals (most commonly a combination of tenofovir and emtricitabine) are taken by individuals at substantial risk of HIV acquisition to prevent infection. There were 492 new HIV diagnoses notified in Ireland in 2017, giving rise to a notification rate of 10.3 per 100,000 population (based on Irish census data). The National Sexual Health Strategy 2015–2020 has called for a comprehensive restructuring of its HIV prevention initiatives, with Priority Action 3 calling for “the appropriate use of antiretroviral therapy in HIV prevention”.
This research was carried out in accordance with HIQA’s guidelines for the conduct of HTAs. In summary, the following took place:
- The Terms of Reference of the HTA were agreed between HIQA, the Department of Health and the Clinical Lead in Sexual Health (HSE).
- An Expert Advisory Group was convened comprising representation from relevant stakeholders, including the Department of Health, the HSE, the Health Protection Surveillance Centre, clinicians with specialist expertise in HIV and sexual health, the National Centre for Pharmacoeconomics, a medical ethicist and relevant patient advocacy groups. An Evaluation Team was appointed comprising HIQA staff.
- The epidemiology of HIV in Ireland was assessed.
- A systematic review and meta-analysis of randomised controlled trials (RCTs) was carried out to summarise the available evidence on the efficacy and safety of PrEP.
- A systematic review was undertaken to summarise the available cost-effectiveness evidence relating to oral PrEP.
- An economic model was developed to estimate the cost-effectiveness of the proposed PrEP programme in the Irish setting from the perspective of the public health and social care system.
- A budget impact analysis reporting the incremental costs associated with the proposed PrEP programme over a one and five-year time horizon was also performed.
- An analysis of the organisational, social and ethical implications was undertaken with a view to identifying broader considerations that may influence decision-making.
- The complete draft report was reviewed by the Expert Advisory Group, before being made available for targeted and public consultation to give interested parties an opportunity to comment on the draft before it was finalised.
- A final draft of the report, including a report on the results of the public consultation, was compiled and submitted to the Board of HIQA for approval.
- Following its approval, the completed assessment was submitted to the Minister for Health and the HSE as advice and published on the HIQA website.
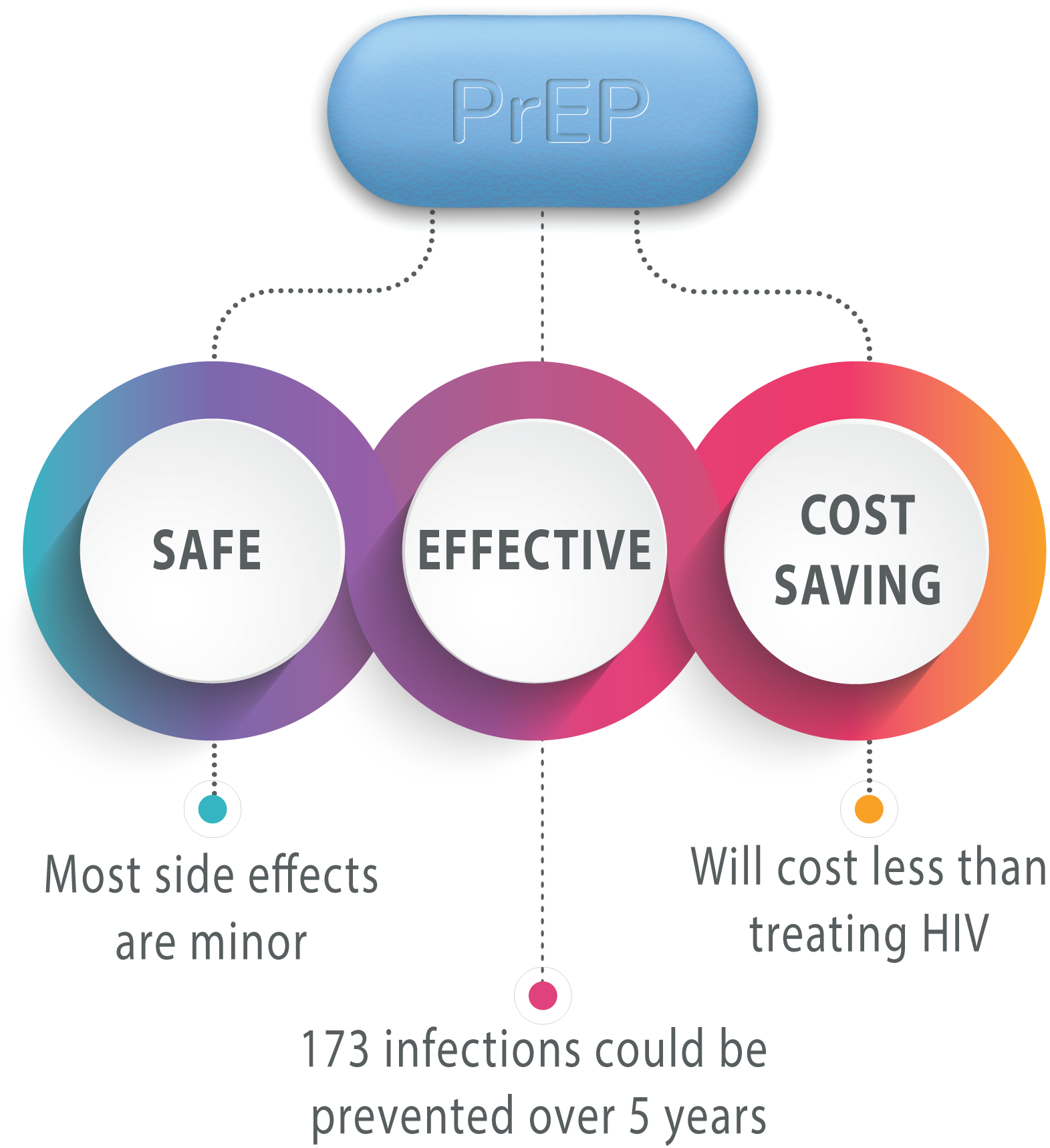
- PrEP is safe and highly effective at preventing sexual acquisition of HIV infection in gay, bisexual and other men who have sex with men and in HIV-negative partners of serodifferent couples (where one partner is HIV-negative and the other is HIV-positive and not on effective antiretroviral therapy). PrEP effectiveness is highly dependent on adequate medication adherence.
- A PrEP programme would provide medication along with holistic assessment, monitoring and frequent testing for HIV and other STIs, advice on safer sex practices, medication adherence support and counselling for individuals at substantial risk of infection.
- An economic model was developed to estimate the costs and consequences of providing a PrEP programme to individuals at substantial risk of infection. PrEP was found to be more effective and less costly than not providing PrEP.
- The incremental budget impact of a national PrEP programme is €1.5m in the first year and €5.4m over five years. Overall, 173 HIV infections are estimated to be averted in the first five years.
- The budget impact is limited to the additional cost to provide a PrEP programme. However, the existing capacity constraints, staffing and infrastructural issues of public STI services should be acknowledged. Significant investment in the broader service may be necessary to support the provision of a safe, sustainable and equitable PrEP programme.